Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Endovenous Laser Ablation of the Surface Tributaries of the Main Veins
*Corresponding author:Sergei Derkachev, Head of Ambulatory Surgery Department Clinic of high medical technologies named after N.I. Pirogov of St. Petersburg State University, 198020, Saint-Petersburg, Staro-Petergofskyi pr
Received:January 09, 2023; Published:January 23, 2023
DOI: 10.34297/AJBSR.2023.17.002412
Abstract
Using optical methods and ultrasound diagnostics, we studied thermal and transport processes caused by boiling of blood inside pathologically altered surface tributaries of the main veins under the action of continuous near-IR laser radiation (1.47 - 1.56 microns) delivered via an end-type optical fiber. These processes were investigated during the experiments with non-aerated water, as well as the clinical procedure of laser endovenous thermal ablation of the surface tributaries of the lower extremities’ main veins. In the proposed approach, thermal modification (ablation and fibrosis) of pathologically altered vessels occurs as a result of blood boiling in the vein lumen with the generation of heated flooded jets that move from the heat source (the end of the fiber), transferring the heated fluid to the venous walls. Jets, as a phenomenon of forced convection, provide the fastest possible transfer of the heat of boiling blood to the endothelial lining and intima of veins.
Keywords: Cell free fetal DNA, Apoptotic fetal DNA, Intrauterine death, Oli gohydaraminos, Multiple trisomes, Miscarriages
Abbreviations:Lasers; Optical fiber; Laser-induced boiling; Heat transfer; Biological fluid; Endovascular ablation; Endovenous coagulation
Introduction
The technology of endovenous laser thermal ablation of veins (ELTA), as follows from monograph [1], is experiencing the last, 7th stage of its life cycle, which is inherent in every medical technology, according to McKinley J [2]. During this period, ELTA is being replaced by new improved technologies of thermal ablation that do not require tumescent anesthesia, for instance, such as non-thermal non-tumescent (NTNT) techniques: “adhesive” and “mechanochemical” ablation [1]. However, if we take a broader look and make use of the fundamental results obtained in thermophysics in the study of liquid heating using concentrated heat sources, which, in particular, is the working tip of an optical fiber immersed in blood, it turns out that the methods of thermal ablation of blood vessels have not exhausted their potential at all. These methods are universal and can be used in the treatment of other diseases, not related to vascular diseases. Therefore, the huge material accumulated by surgeons-phlebologists in the field of ELTA will undoubtedly be in demand regardless of the development of other or new areas of surgical treatment of varicose veins.
Endovenous laser ablation is a surgical treatment method based on the destructive effect of heat, in which a laser device performs the function of a heating device, and the distal quartz or sometimes sapphire tip of the optical fiber performs the function of a heating element. The optical fiber is inserted into the lumen of the vessel, coming into contact with blood. Laser radiation propagates through the fiber, and, upon leaving the fiber, is converted into heat, which warms us the blood - a liquid with a high heat capacity, which makes it possible to transfer heat to the walls of a pathologically altered vessel very efficiently by convection [3]. The process of convective heat transfer is the fastest heat transfer process; however, it increases manifold (more than a hundred times) when the liquid boils [4].
The boiling of blood in the vein near the tip of the fiber is accompanied by the formation of controllable heated jets [5- 8]. The heat obtained as a result of laser radiation conversion is concentrated in these jets. Therefore, by regulating the heat flows concentrated in the jets and, consequently, the heat supply to the pathologically altered walls of varicose veins, it is possible to control the process of their thermal modification. This circumstance plays an important role in endovascular laser ablation of superficial veins, when uncontrolled heat flows from boiled blood can lead to burns of perivascular tissues: lymphatic and blood vessels, nerves, subcutaneous fat and skin.
In this regard, the paper proposes a modified method of endovascular laser ablation of the surface tributaries of the main veins of lower extremities. This method is called “skewer ablation” and is based on the results of the studies which show that by changing the geometry of the tip of an optical fiber immersed in a liquid, it is possible to change the heat flux, which is concentrated in jets generated during laser-induced boiling of an underheated liquid [8]. By varying the heat flow, it is possible to avoid overheating of perivascular tissues, which, in particular, is a problem of the traditional method of laser treatment of superficial tributaries of the main veins of lower extremities, in which optical fibers with a “radial” nozzle are used.
The paper investigates a modified technology of endovenous laser ablation of superficially located tributaries of the main veins, which uses modified optical fibers, the quartz tip of which has been pre-sharpened into a cone shape. The tip of the optical fiber, sharpened in the form of a cone, allows piercing a convoluted vein with the sharp tip of the cone, stringing it on the light guide as if on a skewer and thus conducting skewer ablation of the vein.
The aim of this study is to provide a scientific justification for the safe and atraumatic endovenous laser ablation of superficially located varicose suprafascial tributaries of the main veins by skewer ablation using modified optical fiber.
Materials and Methods
In a model physical experiment, a semiconductor laser with a wavelength of 1.47 microns and quartz-quartz polymer fibers with a diameter of 400 microns in quartz were used. The experiments were carried out in non-aerated water at a temperature of 22°C in a cell with dimensions of 12.5 X 2.3 X 4.1 cm using a FASTCAM SA3 high-speed video camera with a shooting speed of up to 500,000 frames per second. The shadow pattern of liquid boiling near the tip of the optical fiber “to the lumen” was investigated. To control the radiation power, a Field Master power meter with an LM-10 HTTP (Coherent) measuring head was used. All experiments were conducted at room temperature.
The process of blood boiling in the vein was evaluated intraoperatively using an ultrasound device: SonoScape with a 12L-A linear sensor in B–mode. This mode allows visualizing the process of formation of vapor-gas bubbles during the thermal ablation of veins. To assess the blood flow in the active zone of the process, the color duplex and triplex scanning mode with Doppler effect was also used.
The thermal ablation of varicose veins in the clinic was performed using a diode laser device with a wavelength of 1.47 microns. The diameter of the ablated veins before the procedure varied between 0.4 - 1.0 cm. An optical fiber with a 1 mm diameter was used, the distal quartz tip of which had been ground into a cone with an angle at the top of 600.
A puncture of the skin with an 18 G needle was performed over a pronounced convoluted vein. Through this puncture, controlled by ultrasound scanning, a puncture of the distal section of the altered vein was performed with a 60-degree sharpened tip of an optical fiber. Next, the light guide moved in the direction from the main vein, along the modified vein, for its entire length. The vein was impaled on the light guide and positioned (skewer-ablation) (Figure 1).
Between the anterior wall of the vein and the skin, tumescent anesthesia (not tight or tense) is performed with a cooled solution - 50 ml of 4% soda solution (sodium bicarbonate) and 20 ml of 2% lidocaine are added to 1liter of saline solution (0.9% sodium chloride solution). The solution is not injected under the vein. In this case, the saline solution does not compress the vein around the light guide. After that, laser radiation with a wavelength of 1.47 microns and a power of 6 - 8 watts is fed into the fiber and the fiber begins to move inside the vein lumen to the skin puncture site, bringing it out.
The traction is performed manually at a speed, the choice of which is focused on closing the walls of the vein around the light guide. After the procedure, the puncture sites are sealed with an aseptic sticker and a compression knitwear of the 2nd compression class is put on the lower extremities.
The study used intraoperative diagnostic biopsy of the area ablated by skewer ablation of the intersaphenic vein from the inner surface of the upper third of the right tibia. The histological examination of the circular vein section was carried out with 100- fold and 40-fold magnification using hematoxylin-eosin staining of the drug.
From September 2020 to November 2022 256 patients had 288 skewer obliteration operations in the clinic. In the group, 179 (62%) operations were performed on women (aged 33 to 74 years), 109 (38%) operations were performed on men (aged 27 to 76 years). In order to determine the depth of thermal damage to the veins during the operation, a biopsy of a vein obliterated by laser skewer obliteration was taken from 11 patients (8 women and 3 men aged 45-65).
Results
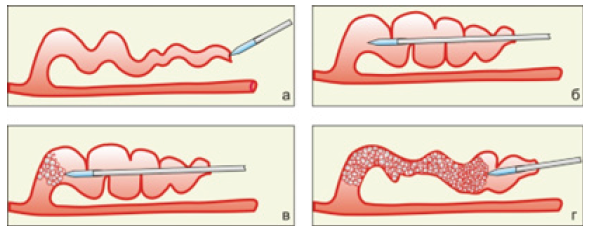
Figure 2A-2C shows the fragments of volumetric boiling water underheated to saturation temperature with the formation of heated water jets, propagating from the tips located in an open cuvette of laser optical fibers immersed in water. The figures show the direction of the jets and their corresponding heat flows, which propagate from the tips of optical fibers with different geometries. Figure 2A shows the heat flow from the optical fiber with a “radial” nozzle, Figure 2B- from the end-type optical fiber, Figure 2C- from the tip of the optical fiber sharpened like a cone.
It is clearly seen that from the fiber with a “radial nozzle” the heated jets propagate perpendicular to the axis of the fiber in the plane of the circle radially (Figure 2A), whereas from the fiber of the end type and the one sharpened like a cone, the jets move in the same direction - along the axis of the fiber forward from the end and the tip of the cone of the light guide (Figure 2B & Figure 2C). Accordingly, the heat flows concentrated in the jets also move in different directions perpendicular to the fiber with a “radial” nozzle and the fibers of the end and cone-sharpened type.
The process of jet formation during the boiling underheated to the saturation temperature is discussed in [6,7] and shown in Figure 3. Figure 3 illustrates the boiling process - the growth and collapse of one bubble under the action of laser heating of water in the vicinity of the tip of the end-type optical fiber (Figure 3A), the fiber sharpened like a cone (Figure 3B) and the fiber with a “radial” nozzle (Figure 3C).
As a result of the absorption of continuous laser radiation with a wavelength of 1.47 microns and a power of 7 watts in water near the tips of optical fibers, the water heats up and boils. The collapse of the bubble at the tip of the optical fibers leads to the generation of heated jets that transfer heat to the intima of the vein at high speed, leading to thermal modification (irreversible denaturation of proteins) of the venous wall.
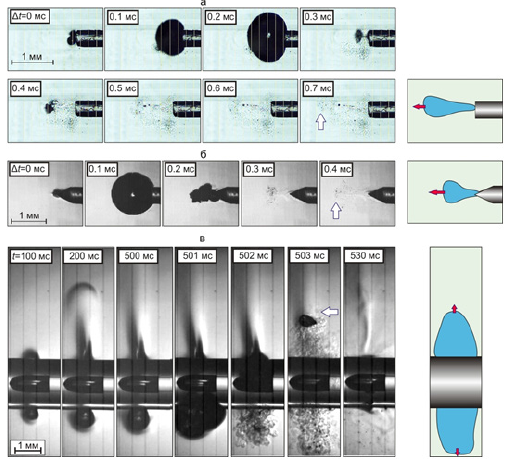
Figure 3:“A” The growth and collapse of the vapor bubble during underheated bulk boiling of non-deaerated water under the action of continuous laser radiation with a wavelength of 1.47 μm with a power of 7 W, passing through the optical fiber with a diameter of 400 mm on quartz, the tip of which is shaped like the end of the cylinder (fiber socket type) (a) like a cone (b) and on the “radial» head of the fiber (c). “B”. Shooting speed 250,000 fps. Frame duration 1/100000 s. The optical fibers are arranged horizontally in the cuvette.
The process of ultrasonic navigation, during the ELTA procedure, is directly related to the control of the boiling zone of blood at the end of the light guide with the formation of a hyperechoic vapor-gas mass in the lumen of the vessel and compression of the venous wall. Figure 4 shows ultrasound images of various stages of laser coagulation using various light guides with the effect of bubble formation and assessment of blood flow in Doppler mode.
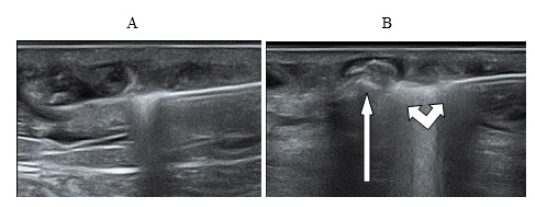
Figure 4:(A) The light guide in the lumen of the vein; (B) Ultrasound picture of the generation of the first bubble during thermocoagulation using laser radiation with a wavelength 1.47 microns and power 7.5 watts.
Ultrasound observation in Doppler mode allows us to record a slowdown in blood flow from the main vein to the surface inflow sometime after the start of the procedure. Later on, the blood flow stops completely. During the procedure, one can observe an active movement of a vapor-gas substrate (foam) in the lumen of the main vein, which spreads through the lumen of the ablated vein and fills the “knee bends” - the convoluted parts of the vessel with varicose extensions [9].
During the boiling process, the blood foams, spreading along the vein and forming a foamy occlusion of the vessel [9]. The “life time” of the foam is less than 24 hours, because on the next day, during a control study in the lumen of ablated veins, the presence of a hyperechoic substrate with gas inclusions is already not detected. A day after the skewer ablation procedure, the vein becomes ablated: the lumen of the vessel is filled with heterogeneous detritus, the walls of the vein are not thickened, there is no blood flow, perivascular tissues have no signs of compaction/inflammation.
The histological examination of the intersaphenic vein area (between the great saphenous vein and the perforant of the medial group) from one third of the lower leg, ablated by laser skewer ablation, shows that the endothelial lining is not determined circularly throughout the inner surface of the vein (Figure 5(1)). The inner layer (1/4 of the vein wall) is compacted, with deformed muscle fibers, karyopycnosis and basophilia of the interstitial substance (Figure 5(2)). There is a parietal fibrin thrombus in the lumen of the vein, without any signs of a cellular reaction (segmented leukocytes are absent) (Figure 5(3)). Perifocally, adventitia is moderately edematous, with focal hemorrhages and single segmented leukocytes.
Conclusion: The histological picture is characteristic of coagulation necrosis of the inner surface of the vein wall with a thickness of ~ 1/4 of the wall.
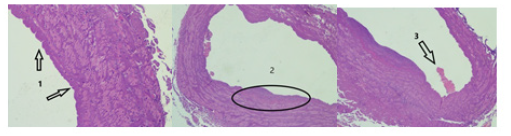
Figure 5:The result of the histological examination of the intersaphenous vein area (between the great saphenous vein and the perforant of the medial group) with 1/3 of the lower leg, ablated by laser skewer. Staining with hematoxylin-eosin. Frame 1 - magnification x 100; frame 2, 3 - x 40.
Figures 6 & 7: the ultrasound picture of the ablated vein 2 weeks after the surgery (Figure 6) and 7 months after the operation (Figure 7).
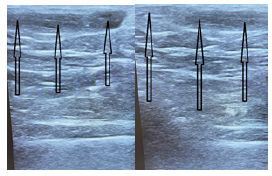
Figure 6:The view of the skewer ablated vein 2 weeks after surgery. The arrows indicate the ablated vein.
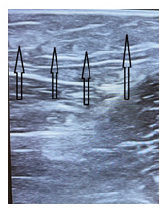
Figure 7:It shows that the soft tissues around the ablated vein are not damaged, there is a clear structuring of tissues without an inflammatory component.
123 skewer ablation operations were performed in 109 patients in the clinic. In the group, 75 operations were performed in women (aged 33 - 74 years) and 48 in men (aged 27 - 76). Figures 8 & 9 show clinical examples.
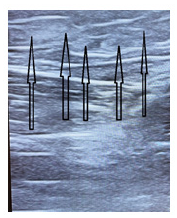
Figure 8:The view of the skewer ablated vein 7 months after the surgery. The arrows indicate the fibrous tissue at the site of the ablated vein.
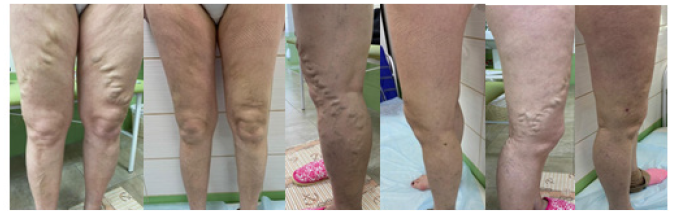
Patient I. 60 years old was diagnosed with varicose veins of the lower extremities without ulcers or inflammation (I83.9). She had been suffering from varicose veins for more than 10 years. Previously, she had not sought medical help, taking venotonics periodically, but with no result. The duplex scanning of the lower extremities veins revealed the failure of the ostial valve of the saphenofemoral fistula on both sides, the valvular insufficiency of the great saphenous vein in the upper third of the thigh and the anterior veins surrounding the thigh on the thigh and the shin. During the examination no ultrasound signs of deep thrombosis and thrombophlebitis of the superficial veins of the left and right lower extremities were detected.
The ELTA operation of the trunk of the great saphenous vein on the right was performed in a standard way under Sol tumescent anesthesia. Lidocaini 0.1% - 250 ml using “radial” optical fiber and continuous laser radiation with a wavelength of 1.47 microns and power of 8.5 watts. At the same time, the anterior vein surrounding the femur was treated by skewer ablation.
After the manipulation, an aseptic bandage was applied to the puncture sites, and a compression jersey was put on the treated leg. The patient was discharged from the department on the same day. A week later, the patient’s left lower limb was operated in the same way. The patient, wearing a compression knitwear, was released home on the same day.
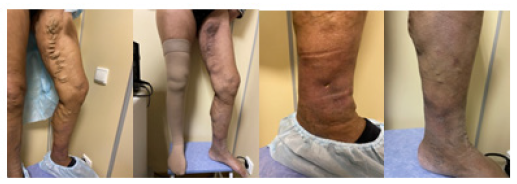
One month after the operation, the examination revealed the absence of dilated veins and hyperpigmentation and a good cosmetic effect (Figure 8). The ultrasound duplex scanning showed a complete ablation of the trunk of the great saphenous vein and convoluted suprafascial tributaries, with no signs of recanalization.
Patient K., 72, was diagnosed with varicose veins with ulcers and inflammation (I83.2). She had been ill for more than 10 years. During this time, following the expansion of the diameter and an increase in the number of dilated veins of the lower extremities, swelling and heaviness in the legs had appeared. In May 2021, ulcers appeared on the left shin, which she unsuccessfully treated with ointment dressings and compression knitwear were. The duplex scanning revealed no signs of deep thrombosis and thrombophlebitis of the superficial veins of the left and right lower extremities but showed that the saphenofemoral anastomosis on the left was not consistent – the reflux in the apical part went into a pronounced convoluted superficial accessory vein, annularly closing in the upper third of the thigh on the trunk of the great saphenous vein. Then it again went along the trunk to the middle third of the lower leg, then - into the anterior and posterior accessory and intersaphenic veins and drained into the deep venous system through the perforant veins of all the shin groups, as well as into the short saphenous vein, causing valvular insufficiency in the middle third and lower third of the shin.
On 19.10.2021 the standard ELTA method was used to operate the patient’s great saphenous vein of the upper third of the left shin under the control of duplex scanning. At the same time, the superficial accessory subcutaneous vein of the left lower limb in the middle third of the thigh was operated by the “Skewer” method. Under local anesthesia with the control of duplex scanning, the “Skewer” light guide was inserted into the vein and carried to the saphenofemoral junction, where it was positioned. Endovenous laser ablation was performed at a continuous laser radiation power of 6.5 W in manual traction mode at a speed of 1 mm/sec.
The postoperative period proceeded without any peculiarities. The patient, wearing a compression knitwear, was discharged on the same day.
The examination conducted 2 weeks later showed that the ulcers were epithelized and lip dermatosclerosis had decreased. A satisfactory cosmetic effect was noticed. Ultrasound duplex scanning at the time of the study showed no signs of deep thrombosis and thrombophlebitis of the superficial veins of the left and right lower extremities, as well as no signs of recanalization. A complete ablation of the trunk of the great saphenous vein and convoluted suprafascial tributaries was noted Figure 9 & Figure 10.
Discussion
The paper provides a physical substantiation of a new technology for the treatment of varicose veins - superficially located tributaries of the main veins by endovascular laser ablation, which is devoid of the disadvantages of miniflebectomy and sclerobliteration, namely, the painfulness of the procedure, damage to perivascular vessels, nerves, subcutaneous fat, postoperative hematomas, infection of postoperative wounds, scars in places of micro-incisions, frequent relapses of the disease, the formation of “coagules” inside the lumen of the vein, hyperpigmentation and necrosis of the skin [10-20]. The new ELTA technology does not use conventional optical fibers with a “radial” nozzle and end-type fibers. For the treatment of varicose veins - superficially located tributaries of the main veins, since the use of “radial” optical fibers leads to overheating and burning of perivascular tissues, and the use of end-type fibers is difficult due to the high degree of tortuosity of surface veins.
In this regard, the “Skewer” technology was proposed, which is based on the use of a cone-sharpened tip of the end fiber, with which a convoluted surface vein is strung on the fiber, like on a skewer. The aim of the study is to show that heat flows arising from the boiling of blood on laser heating elements made in the form of a cone-sharpened tip of the end fiber cannot lead to overheating and burning of perivascular tissues, as is the case of a “radial” nozzle fiber.
The lumen of the ablated vein is compressed due to the evaporation of the liquid component of the blood, denaturation of blood proteins and endothelium; the lumen of the vein is filled with protein foam and detritus. Over time, the vein becomes scarred. The proposed method of laser ablation of strongly convoluted veins does not require additional tools (conductors, catheters or introducers, etc.), since the passage of the light guide inside the vein and in the perivascular tissues is carried out via the compacted working part of the light guide, pointed at an angle of 60 degrees; it ensures a low injury rate and a good cosmetic effect, reduces the duration of treatment and recovery of patients, as well as the occurrence of hemorrhages and hematomas.
The research conducted in this way provides a perfect reason for implementing the “Skewer” technology in medical practice.
We studied heat flows concentrated in flooded jets, which are generated when water boils on laser heating elements - the tips of end-, radial-, and cone-type optical fibers. Laser radiation with a wavelength of 1.47 microns was used, which is strongly absorbed in water with a coefficient of cm-1, so the water in the vicinity of the optical fiber tip boils quickly with the formation of cumulative flooded jets, the temperature of which is close to the boiling point. Boiling with the formation of jets is a forced convection mode in which heat is mainly concentrated in the jet stream. It has been experimentally shown that when using optical fiber with a “radial” nozzle, generated heat flows are directed to the venous walls at right angles and thus transmit maximum heat to the walls. This heat is quickly transferred to nearby anatomical structures, in particular, perivascular soft tissues and skin, and causes their heating and burns. When using end-type and cone-sharpened fiber, heat flows are directed along the fiber axis deep into the lumen of the varicose vein. It is important that in this case, changing the geometry of the end of the fiber from a cylinder flat in section to a cone does not lead to a change in the heat flow direction.
Reducing the power of laser radiation cannot improve this situation, since blood boiling is of a threshold nature. When the radiation power is below the threshold, blood boiling, and, consequently, vein ablation, simply does not occur, whereas when the radiation power is above the threshold, the boiling process, which causes the blood to warm up to 1000C, already weakly depends on the radiation power. In this case, everything is determined by the heat flow direction.
The two-phase flows of heated blood with bubbles generated via underheated boiling are clearly observed during ultrasoundassociated ELTA manipulations. When a “radial” nozzle is used, these flows are directed strictly to the venous walls and then spread along the intima, whereas they spread deep into the vein from the “end” type fibers and transfer heat to the venous walls for a longer length, but less intensively [11,21].
Conclusion
The paper provides a physical substantiation of a new original technique of surgical treatment of convoluted varicose veins of the superficial tributaries of the main veins by endovasal laser obliteration. The use of a special optical fiber, with a cone-wise sharpened quartz tip, allows stringing a strongly twisted vein on the fiber as if it were a skewer, carrying out laser obliteration of the vein. The flows of heated blood, concentrated in jets generated by boiling blood at the tip of the optical fiber (laser heating element), spread along the inner surface of the vessel, repeating all its bends and protrusions, evenly warm the intima without deep damage to venous wall and paravasal tissues, leading to thermal degeneration of the varicose vein, which is subsequently replaced by fibrous tissue. The proposed method of laser obliteration of highly convoluted veins does not require additional tools (conductors, catheters, introducers, phlebectomy hooks, etc.), since the light guide inside the vein and in paravasal tissues is conducted via the working part of the light guide pointed at an angle of 60 degrees. The injury level is low; good cosmetic effect is produced, which reduces the duration of treatment and recovery of patients, as well as the occurrence of hemorrhages and hematomas.
References
- Stoiko Yu M, Mazaishvili KV (2020) Endovenous laser ablation.
- Wright J (2005) Hormone replacement therapy: An example of McKinlay’s theory on the seven stages of medical innovation. J Clin Nurs 14(9): 1090-1097.
- Chudnovskii VM, Yusupov VI, Zakharkina OL, Ignatieva NYu, Zhigarkov VS, et al. (2016) Contribution of laser-induced gas-vapor-liquid dynamics to the mechanism of endovenous laser ablation. Sovremennye tehnologii v medicine 8(2): 6-13.
- Nesis EI (1973) Boiling of liquids. M Nauka pp. 280.
- Yusupov VI, Chudnovskii VM, Bagratashvili VN (2011) Laser-induced hydrodynamics in water-saturated biotissues: 2. Effect on delivery fiber. Laser Phys 21(7): 1230-1234.
- Chudnovskii VM, Levin AA, Yusupov VI, Guzev MA, Chernov AA (2020) The formation of a cumulative jet during the collapse of a vapor bubble in a subcooled liquid formed as a result of laser heating. International Journal of Heat and Mass Transfer 150(2020): 119286.
- Fursenko RV, Chudnovskii VM, Minaev SS, Okajima J (2020) Mechanism of high velocity jet formation after a gas bubble collapse near the microfiber immersed in a liquid. International Journal of Heat and Mass Transfer 163(2020).
- Chudnovskii VM, Yusupov VI, Dydykin AV, Nevozhay VI, Kiselev AYu, et al. (2017) Laser-induced boiling of biological fluids in medical technologies. Quantum electronics 4(47): 361.
- Chudnovskii VM, Makhovskaya TG, Major AYu, Yusupov VI, Nevozhay VI, et al. (2018) Foaming of blood in the mechanism of endovascular laser ablation. Phlebology 4: 261-269.
- Deng R, He Y, Qin Y, Chen Q, Chen L (2012) Measuring pure water absorption coefficient in the near-infrared spectrum (900 - 2500 nm). Yaogan Xuebao - Journal of Remote Sensing 16(1): 192-206.
- Chudnovskii VM, Makhovskaya TG, Mayor AYu, Yusupov VI, Nevozhai VI, et al. (2018) The Role of Blood Foaming in the Mechanism of endovascular Laser Ablation. Flebologiya 4: 261-269.
- Van der Geld CW, van den Bos RR, van Ruijven PW, Nijsten T, Neumann HA, et al. (2010) The heat-pipe resembling action of boiling bubbles in endovenous laser ablation. Lasers Med Sci 25(6): 907-909.
- Baccaglini U (1998) Sclerotherapy of varicose veins of the lower extremities. Phlebolymphology 8: 8-12.
- Bukina OV (2010) Does vein thrombosis develop after the introduction of a sclerosing drug? Phlebology p. 28-33.
- Kirienko AI (2004) Compression sclerotherapy (a practical guide for doctors). Kirienko AI & Bogachev VYu (Eds.), The Publishing house of the National Academy of Sciences named after A.N. Bakulev RAMS, p. 40.
- Krylov AY (2000) Compression sclerotherapy in the complex treatment of patients with varicose veins. Angiology and vascular surgery 6(1): 49-54.
- Zolotukhin IA, Seliverstov EI, Zakharova EA, Kirienko AI (2016) The isolated removal of tributaries of the incompetent great saphenous vein leads to the restoration of the function of its valves. Phlebology 10(1): 8-18.
- Russian clinical guidelines for the diagnosis and treatment of chronic venous diseases. Phlebology 12(3): 146-240.
- Baeshko AA (2012) Foam sclerotherapy: the history of development and modern data. News of surgery 20(4): 101-110.
- Savelyeva VSM (2001) Phlebology. A guide for doctors. Publishing House "Medicine".
- Belikov AV, Skrypnik AV (2018) Soft Tissue Cutting Efficiency by 980nm Laser with Carbon-, Erbium-, and Titanium-Doped Optothermal Fiber Converters. Lasers in Surgery and Medicine 9999: 1-16.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.